Trump Promised to ‘Save Vaping.’ Does His New FDA Head Agree?
FDA Admin. Makary’s comments on youth vaping clash with data & Trump’s pledge to “save vaping,” raising doubts about flavored e-cigarette policy.
During his 2024 presidential campaign, Donald Trump made a clear promise to the vaping community: he “saved flavored vaping” during his first term and would “save vaping again.” This pledge resonated with many adult vapers and former smokers who rely on flavored e-cigarettes, a category largely restricted by the Food and Drug Administration (FDA) under the previous administration. The FDA’s de facto ban on vaping products in flavors other than tobacco and menthol has been a contentious issue, as these are often not the preferred choices for adults transitioning away from combustible cigarettes. However, recent comments from Marty Makary, President Trump’s newly appointed FDA Administrator, have cast significant doubt on whether the agency is prepared to align with this campaign commitment, particularly concerning flavored products.
Makary’s Alarm: Exaggerating Youth Vaping Rates?
Testifying before a Senate appropriations committee last week, FDA Administrator Makary painted a stark picture of youth vaping, a narrative that has long been the primary justification for the FDA’s stringent restrictions on e-cigarette flavors. In comments he later highlighted on his official X (formerly Twitter) account, Makary asserted that “there are high schools in America now where kids are saying half of the kids in high school are addicted to these vaping products.”
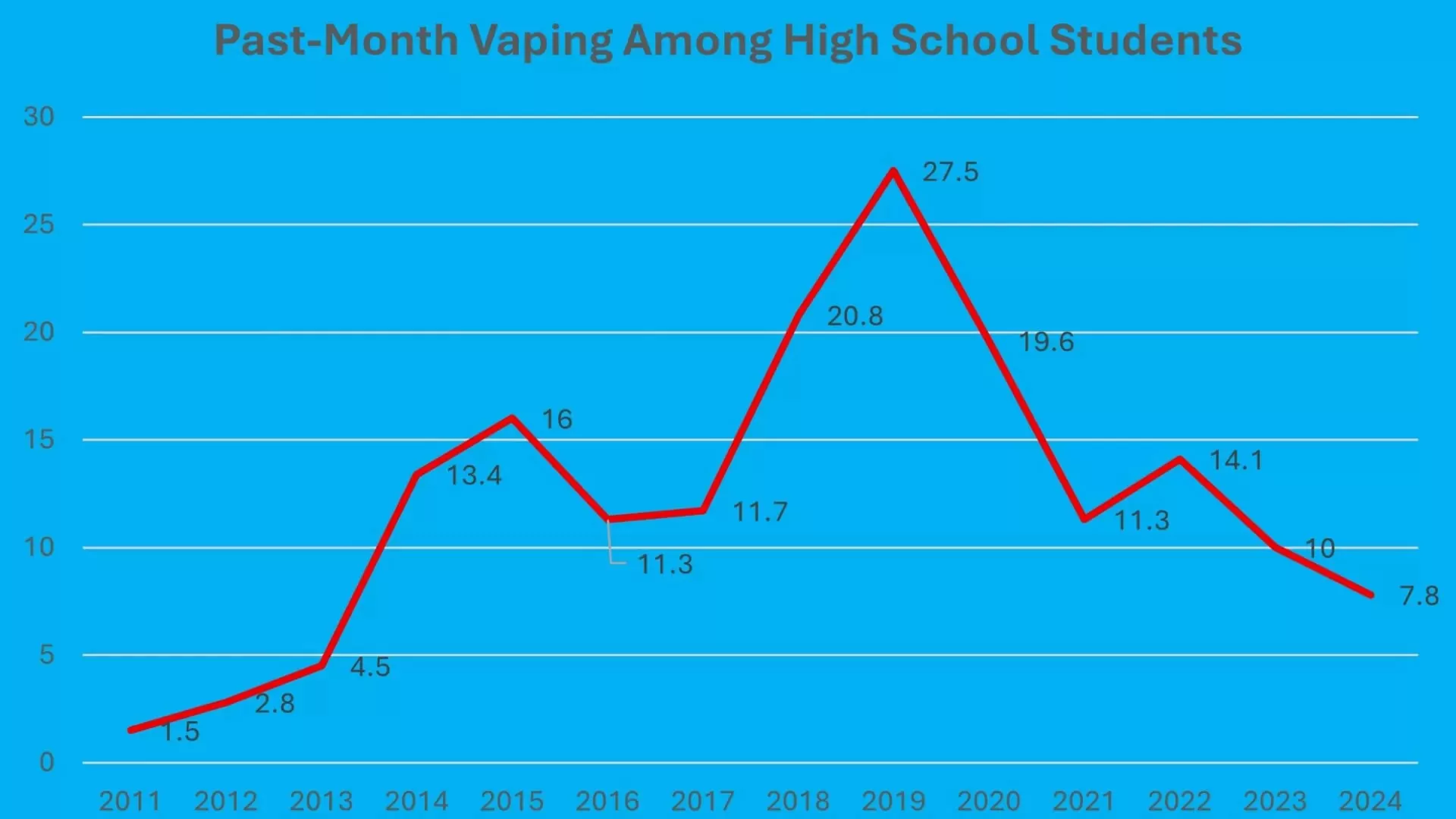
This statement, suggesting a 50% addiction rate in some high schools, stands in sharp contrast to the latest official data from the National Youth Tobacco Survey (NYTS), conducted annually by the Centers for Disease Control and Prevention (CDC). The 2024 NYTS found that 7.8 percent of high school students reported using e-cigarettes in the past 30 days. Makary’s figure is more than six times this national prevalence of recent use. If we assume, based on the 2023 NYTS data, that approximately 30 percent of past-month users were daily users (a common proxy for potential nicotine dependence), Makary’s implied addiction rate would be roughly 22 times higher than the estimated rate of daily use among high school students nationwide.
Critically, Makary’s alarming portrayal also overlooks a significant and well-documented trend: underage vaping has fallen dramatically in recent years. The 7.8 percent prevalence of past-month use among high school students in 2024 is down from 10 percent in 2023 and represents a staggering 72 percent decrease from the peak of 27.5 percent recorded in 2019. This crucial context was notably absent from his testimony.
A Senate Hearing: Misleading Narratives and Missed Opportunities
During the Senate hearing, Senator Jeanne Shaheen (D–N.H.), a long-time proponent of stricter e-cigarette regulation, pressed Administrator Makary on the FDA’s efforts to curtail teenage vaping. However, Senator Shaheen’s own statements also obscured the recent significant declines in youth use. “One of the things that I’ve been concerned about for a very long time is the increased use of vaping by young people,” she stated. “The numbers have gone up dramatically in the last 10 years.”
This assertion is not accurate when looking at the most recent data. According to the NYTS, the prevalence of past-month e-cigarette use among high school students in 2024 was less than half the rate recorded in 2015 (when it was 16 percent). Senator Shaheen’s claim also pales in comparison to a statement she made in January 2024, where she incorrectly asserted that “teenage e-cigarette consumption has increased by 1,800% in just the last year.” That 1,800% figure actually referred to the increase in past-month use between 2011 and the peak in 2019. By the time she made that statement in early 2024, rates had already fallen substantially from that 2019 peak, a trend that continued into the 2024 survey results.
Instead of correcting the record or highlighting the significant progress made in reducing youth vaping, Administrator Makary appeared to echo Senator Shaheen’s alarmist tone. “There are kids in America today who are addicted, they know they’re addicted, they come from good families, they’re good kids, and they can’t stop,” he said. “And that is something that we have to address.”
Focus on Enforcement, Silence on Flavors for Adults
Administrator Makary did emphasize a tougher stance on enforcement against illegal, unauthorized e-cigarette products, particularly those originating from China and allegedly designed to appeal to minors. He described previous FDA efforts to block these imports as ineffective, stating, “It’s been a joke, and they’ve been laughing at us… And so we are going to stop that….We’ve got to get serious about them.”
While increased enforcement against illegal and non-compliant products is generally supported, it’s noteworthy that the sharp decline in adolescent vaping occurred during the very period Makary characterized as one of FDA laxity. This apparent contradiction was not addressed, as the downward trend in youth use was not acknowledged by the Administrator during his exchange with Senator Shaheen.
Senator Shaheen, visibly pleased with Makary’s approach, went on to state, “I think we should right-out ban vaping products—for anybody under a certain age, certainly.” Given that federal law already prohibits e-cigarette sales to anyone younger than 21, her comment implies a desire for even more sweeping prohibitions, potentially impacting adult access or raising the age limit further. Administrator Makary offered no comment on the rights of adults to purchase these products, nor did he challenge Senator Shaheen’s dismissal of vaping’s harm reduction potential.
Shaheen remarked, “One of the other selling points that they use is [that] it’s a way to get cigarette users to stop smoking… They can vape, and the harmful impacts are not as serious.” While she framed this as a dubious “selling point,” the FDA itself, under previous administrations, has repeatedly acknowledged the scientific evidence that vaping is a significantly less dangerous alternative to consuming nicotine than smoking combustible cigarettes.
The Flavored Vape Dilemma: Will the FDA Reconsider?
Despite acknowledging the reduced-harm potential, the FDA during the Biden administration consistently refused to authorize marketing applications for vaping products in flavors other than tobacco (and in very limited cases, menthol). This policy effectively created a ban on the vast majority of flavored e-liquids that adult former smokers report preferring and find helpful in their transition away from cigarettes. This FDA stance on flavors was recently challenged in court, but the Supreme Court last month declined to hear the case, thereby allowing the FDA’s marketing denial orders for flavored products to stand. This decision effectively put the responsibility for any change in flavor policy back onto the FDA.
President Trump’s campaign promise to “save flavored vaping” implied a potential shift in this FDA approach, perhaps by directing the agency to reconsider its stance and authorize flavored products that can demonstrate a net benefit to public health, particularly by helping adult smokers quit. However, Administrator Makary’s recent testimony, with its strong emphasis on youth addiction and gross overstatement of current youth vaping prevalence, suggests he may not be inclined to champion such a policy reversal. His focus on a youth “epidemic,” even as data shows significant declines, aligns more closely with the rationale used to justify the existing flavor restrictions rather than a move towards broader adult access to flavored alternatives.
Conclusion: A Disconnect Between Campaign Promises and Agency Rhetoric
The recent statements from FDA Administrator Marty Makary present a concerning disconnect from President Trump’s campaign promises regarding vaping. While a crackdown on illegal, youth-appealing disposable vapes from overseas is a shared goal, the Administrator’s rhetoric on the scale of current youth addiction appears significantly misaligned with the government’s own survey data, which shows a dramatic and sustained decrease in underage vaping over the past five years.
For adult vapers and former smokers who rely on flavored e-cigarettes to remain smoke-free, Makary’s testimony offers little reassurance that the FDA under his leadership will revisit the restrictive flavor policies of the previous administration. If the FDA continues to prioritize an exaggerated perception of youth risk over the potential benefits of flavored vaping products for adult smoking cessation, President Trump’s promise to “save vaping again” may prove difficult to fulfill. The vaping community and public health advocates who support harm reduction will be watching closely to see if the FDA’s actions begin to reflect a more balanced approach that acknowledges both youth protection and the needs of adult smokers seeking safer alternatives.
- Teen Vaping of THC & Synthetic Cannabinoids Surges: Study - July 4, 2025
- UK Wolverhampton Extends “Swap to Stop” Vape Program - July 4, 2025
- Pakistan Halts Vape Crackdown Pending Legislation - July 4, 2025








