FDA Authorizes 6 Vuse Alto Tobacco-Flavored E-Cigarettes
The U.S. Food and Drug Administration (FDA) has authorized the marketing of seven e-cigarette products from R.J. Reynolds Vapor Company, marking a significant milestone in the regulation of electronic nicotine delivery systems (ENDS) in the United States. The agency issued marketing granted orders for the Vuse Alto Power Unit and six Vuse Alto tobacco-flavored pods, which are sealed, pre-filled, and non-refillable, following an extensive scientific review through the premarket tobacco product application (PMTA) pathway.
Authorized Products and Nicotine Strengths
The FDA has given the green light to the following Vuse Alto tobacco-flavored pods:
- Vuse Alto Pod Golden Tobacco 5%
- Vuse Alto Pod Rich Tobacco 5%
- Vuse Alto Pod Golden Tobacco 2.4%
- Vuse Alto Pod Rich Tobacco 2.4%
- Vuse Alto Pod Golden Tobacco 1.8%
- Vuse Alto Pod Rich Tobacco 1.8%
Public Health Standard and Risk-Benefit Analysis
The FDA evaluates PMTAs based on a public health standard that considers, among other factors, the risks and benefits of the product to the population as a whole. After reviewing R.J. Reynolds Vapor Company’s applications, the agency determined that there was sufficient evidence to demonstrate that permitting the marketing of these products would be appropriate for the protection of public health, as required by the 2009 Family Smoking Prevention and Tobacco Control Act.
The applicant demonstrated that these tobacco-flavored products have the potential to provide a benefit to adult smokers that outweighs the risks, including the risk of youth use. However, the FDA emphasizes that this authorization does not mean these products are safe or “FDA approved,” nor does it indicate their appropriateness for marketing as modified risk tobacco products.
Youth Use Concerns and Marketing Restrictions
While the FDA remains concerned about the risk of youth use of all e-cigarettes, data from the 2023 National Youth Tobacco Survey suggests that youth are less likely to use tobacco-flavored e-cigarette products compared to other flavors. Despite Vuse being among the most commonly reported brands used by middle and high school students currently using e-cigarettes, only 6.4% of these students reported using tobacco-flavored products.
To further mitigate youth use of these products, the FDA has placed stringent marketing restrictions on the newly authorized products to prevent youth access and exposure. The agency will closely monitor how these products are marketed and will take appropriate action if the company fails to comply with any applicable statutory or regulatory requirements.
Ongoing Monitoring and Potential Suspension of Authorization
The FDA maintains the right to suspend or withdraw authorization if it determines that continued marketing is no longer appropriate for the protection of public health. This includes situations where there is a notable increase in use of the products among youth or former cigarette users, or a decrease in patterns of complete switching by current cigarette users to the new products.
FDA’s Commitment to Science-Based Review
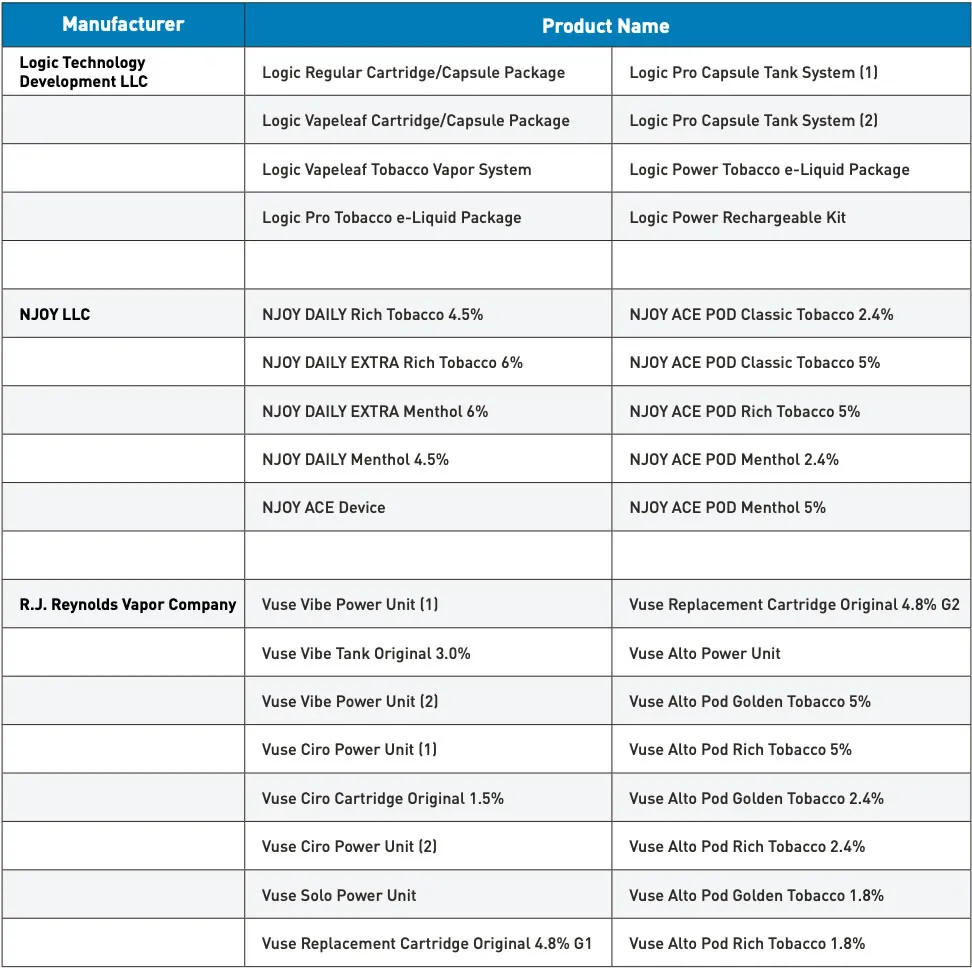
Today’s actions reflect the FDA’s ongoing commitment to ensuring that all new tobacco products marketed in the United States undergo a science-based review and receive the necessary marketing authorizations. To date, the agency has received applications for nearly 27 million deemed products and has made determinations on more than 26 million of those applications. The FDA has authorized 34 e-cigarette products and devices, including the seven authorized today.
Download this item (opens in a new window)
The agency maintains a printable one-page flyer of all authorized e-cigarette products, which are the only e-cigarette products that currently may be lawfully marketed and sold in the United States. Manufacturers, importers, sellers, or distributors of e-cigarettes without the required premarket authorization risk enforcement action. Those seeking a list of legally marketed tobacco products, including e-cigarettes, can visit the FDA’s new Searchable Tobacco Products Database.
Source: FDA Authorizes Marketing of Vuse Alto Tobacco-Flavored E-Cigarette Pods and Accompanying Power Unit
- Bestselling Vapes in UK After Disposable Ban: What to Stock 2025 - August 8, 2025
- Argentina Debates Stricter Vape Laws Amid Prohibition Failures - August 8, 2025
- Nigeria Advocacy Group Urged to Hike Tobacco & Vape Tax by 100% - August 8, 2025