Overview of FDA-Authorized VUSE E-Cigarette Products
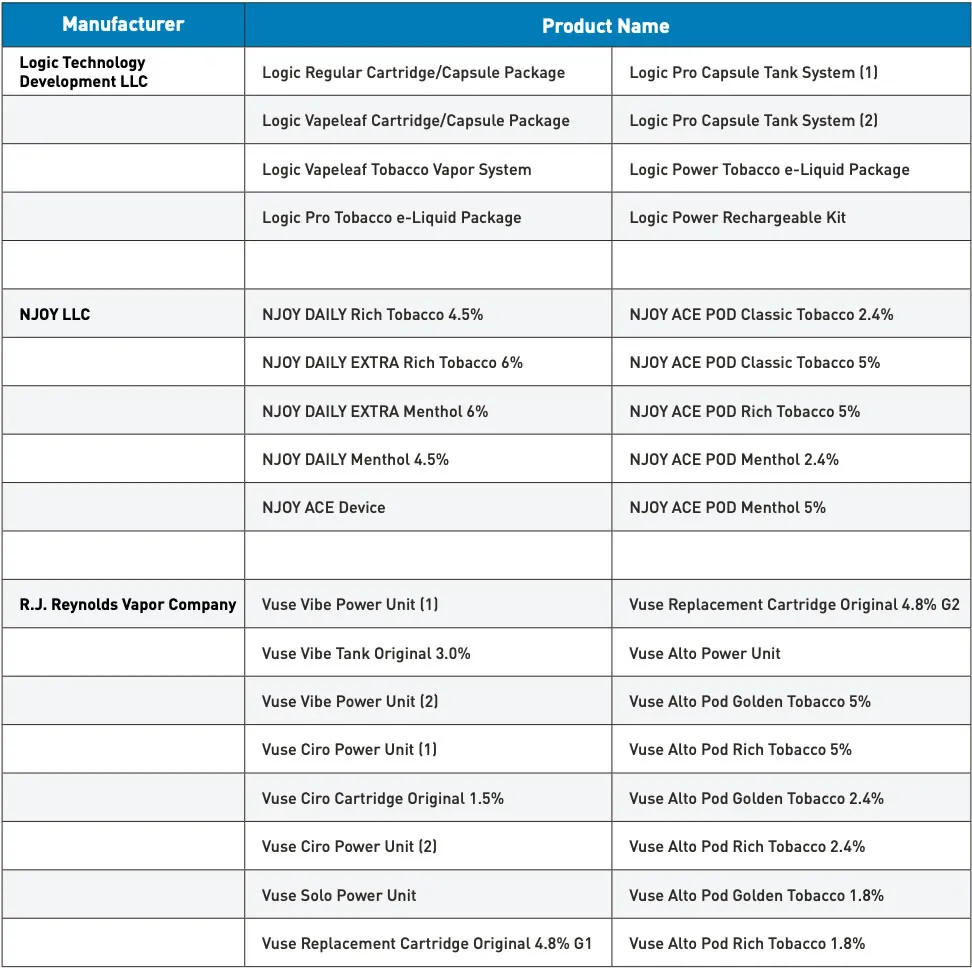
The U.S. Food and Drug Administration (FDA) has granted marketing authorization to RJ Reynolds Vapor Company for an expanded range of Vuse e-cigarette products. This decision, announced on July 18, adds seven new Vuse Alto e-cigarette products to the company’s growing portfolio of FDA-approved offerings. RJ Reynolds Vapor Company, an American subsidiary of British American Tobacco, now boasts the largest portfolio of authorized e-cigarette products in the U.S. market.
Vuse Alto: The Latest in FDA-Authorized E-Cigarettes
The Alto platform consists of the Vuse Alto Power Unit and six closed, pre-filled, and non-refillable Vuse Alto tobacco-flavored pods, utilizing FEELM ceramic coil technology.

- Pod Capacity: 1.8 mL
- Pods:
– Vuse Alto Pod Golden Tobacco 5% (5% nicotine strength)
– Vuse Alto Pod Rich Tobacco 5% (5% nicotine strength)
– Vuse Alto Pod Golden Tobacco 2.4% (2.4% nicotine strength)
– Vuse Alto Pod Rich Tobacco 2.4% (2.4% nicotine strength)
– Vuse Alto Pod Golden Tobacco 1.8% (1.8% nicotine strength)
– Vuse Alto Pod Rich Tobacco 1.8% (1.8% nicotine strength) - Battery Capacity: 350mAh Type-C rechargeable battery
- Draw-activated system, activated without pressing a button
Previously Authorized Vuse Products
Vuse Vibe
The Vibe platform, granted FDA marketing authorization in October 2021, comprises the Vuse Vibe Power Unit (1), Vuse Vibe Power Unit (2), and Vuse Vibe Tank Original 3.0%.

- Tank Capacity: 2mL
- Tank: Vuse Vibe Tank Original 3.0% (3% nicotine strength)
- Battery Capacity: 350mAh Type-C rechargeable battery
- Features: LED indicator light
Vuse Ciro
The Vuse Ciro series received FDA marketing authorization in May 2022. It includes the Vuse Ciro Power Unit (1), Vuse Ciro Power Unit (2), and Vuse Ciro Cartridge Original 1.5%.

- Cartridge Capacity: 0.9mL
- Cartridge: Vuse Ciro Cartridge Original 1.5% (1.5% nicotine strength)
- Battery Capacity: 260mAh Type-C rechargeable battery
- Features: LED indicator light
Vuse Solo
Vuse Solo, granted FDA marketing authorization in October 2021, was the industry’s first electronic nicotine delivery system (ENDS) product to receive premarket tobacco product application (PMTA) clearance and enter the U.S. market. It consists of the Vuse Solo Power Unit, Vuse Replacement Cartridge Original 4.8% G1, and Vuse Replacement Cartridge Original 4.8% G2.

- Cartridge Capacity: 1.2mL
- Cartridges:
– Vuse Replacement Cartridge Original 4.8% G1 (4.8% nicotine strength)
– Vuse Replacement Cartridge Original 4.8% G2 (4.8% nicotine strength) - Battery Capacity: 250mAh Type-C rechargeable battery
- Features: LED indicator light
Adhering to FDA Guidelines
All FDA-authorized Vuse products strictly adhere to a tobacco-only flavor profile, aligning with FDA restrictions on e-cigarette flavors. However, they offer a range of nicotine concentrations to cater to varying user preferences and needs.
Tadeu Marroco, CEO of British American Tobacco, highlighted the significance of this expanded portfolio, stating, “We currently have the largest portfolio of e-cigarette market authorizations available in the U.S., which will help us offer more potential reduced-risk product options to adult smokers.”
This development marks a crucial step in the e-cigarette industry’s evolution. Manufacturers continue to navigate regulatory requirements while addressing adult smokers’ needs for potentially less harmful alternatives to traditional cigarettes.
- Pakistan Halts Vape Crackdown Pending Legislation - July 4, 2025
- Wisconsin New Law Banning Sale of Most Vape Products - July 4, 2025
- Vaping Laws in Oklahoma: A Comprehensive Guide for 2025 - July 3, 2025