46 Countries Have Banned E-Cigarettes and Vapes as of 2025
The global e-cigarette and vape market has experienced a meteoric rise, transforming from a niche curiosity into a multi-billion dollar industry with continued growth projected worldwide. For U.S. businesses looking to tap into this international demand, the opportunities are significant. However, venturing abroad with vape products in 2025 means navigating an increasingly complex and often fragmented landscape of international regulations. Understanding and meticulously adhering to these diverse global vape regulations is not merely advisable; it’s an absolute necessity for successful market entry, sustained operations, and building a trustworthy international brand. This guide provides an essential overview of the global status of e-cigarette bans and regulations as of May 2025, offering critical insights for U.S. businesses aiming for international compliance.
E-cigarettes entered the US market in 2007 and have since evolved into a substantive public health issue, especially regarding adolescents. Monthly sales of these devices surged from 15.5 million units in 2020 to 22.7 million in 2022, reports the Centers for Disease Control and Prevention (CDC). Moreover, e-cigarettes now represent the most widely consumed tobacco product amongst American youth.
Proponents argue e-cigarettes can assist smokers in quitting traditional cigarettes. However, critics counter that the youthful packaging and fruit-flavored varieties specifically target underage users. These factors risk addicting adolescents to nicotine while exposing them to other dangerous chemicals.
A 2023 CDC study discovered approximately 2 million middle and high school students currently use e-cigarettes, representing 7.7% of this population. Over 60% of youth users favored disposable vape brands such as Elf Bar and Esco Bar. In the UK, around 9% of 11-15-year-olds are reported to vape.
In America, the Food and Drug Administration (FDA) regulates e-cigarettes, greenlighting products on a case-by-case basis contingent on the risk of youth uptake. So far, the FDA has approved just 34 e-cigarette products, rendering the thousands of unauthorized items on store shelves technically illegal. Still, enforcement has proven challenging.
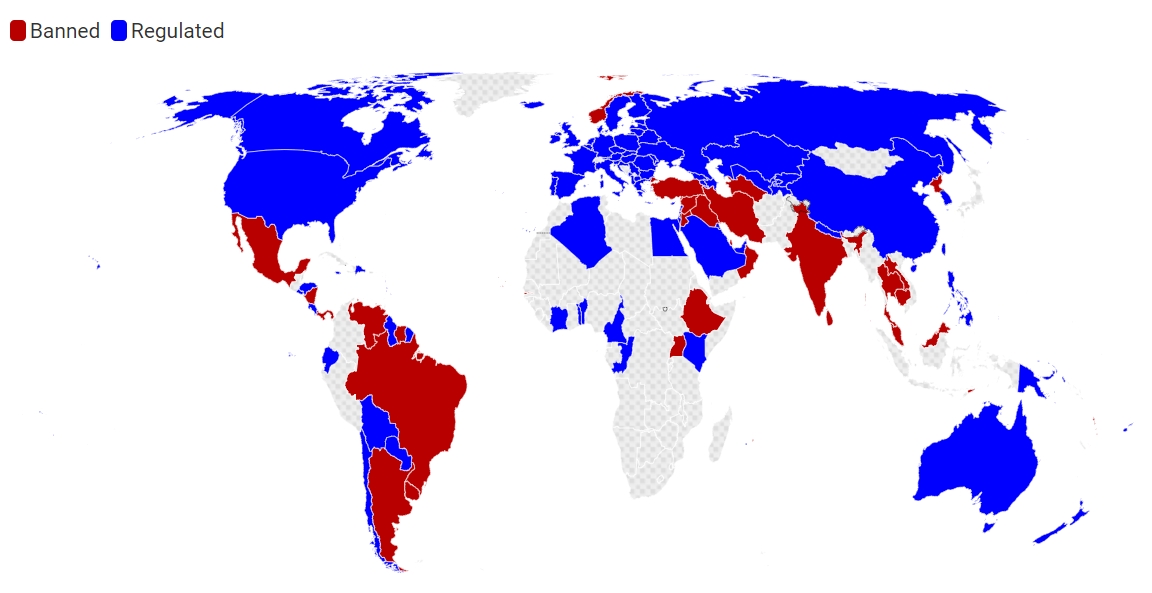
Over 40 Nations Have Banned E-Cigarette Sales (as of May 2025)
According to a May 2025 factsheet by the Global Center for Good Governance in Tobacco Control (GGTC), a significant number of countries have implemented comprehensive bans on the sale and distribution of e-cigarettes. As of this date, 46 countries have such bans in place. This list includes nations across various WHO regions and income levels. For U.S. businesses, these markets are effectively closed for legal sales of e-cigarette products.
The countries reported by GGTC to have e-cigarette sales bans include:
- Argentina
- Bahrain*
- Brazil
- Brunei Darussalam
- Cabo Verde
- Cambodia
- Chile*
- Colombia*
- Democratic People’s Republic of Korea (North Korea)
- Egypt*
- Ethiopia
- Gambia
- India
- Iran (Islamic Republic of)
- Iraq
- Jordan
- Kazakhstan**
- Kuwait*
- Lao People’s Democratic Republic
- Lebanon*
- Malaysia (some states)
- Maldives**
- Marshall Islands**
- Mauritius
- Mexico (despite some court rulings, a ban largely remains enforced)
- Nicaragua
- Norway (though some exceptions for NRT-like products might exist)
- Occupied Palestinian territory
- Oman
- Palau**
- Panama
- Papua New Guinea**
- Qatar
- Singapore
- Sri Lanka
- Suriname
- Syrian Arab Republic
- Thailand
- Timor-Leste
- Turkey
- Turkmenistan
- Uganda
- Uruguay
- Vanuatu
- Venezuela (Bolivarian Republic of)
- Vietnam**
(* Indicates countries listed in a WHO background paper on D-ENDS bans; ** Indicates countries included based on GGTC country contacts and media outlets, with specific year of ban/update often noted, e.g., Kazakhstan (2024), UK (2025 for disposables). It’s important to note the UK’s 2025 ban is specific to disposable vapes, not all e-cigarettes.)
This list also includes jurisdictions like Hong Kong SAR (2022), Macau SAR (2022), and Taiwan ROC (2023) which have banned e-cigarette sales. The increasing number of countries opting for outright sales bans highlights a growing global concern, often centered on preventing youth uptake and addressing unknown long-term health effects.
Ecigator is one of the well-known vape brands spun off from FM Technology Co., Ltd, it’s an ISO-certified disposable vape manufacturer for OEMs, ODMs, and OBM since 2010. The founder team comes from top firms with more than 10 years of experience in the vaping industry and has devoted thousands of hours to providing users with a better and better experience.

18K Disposable Pod Kit
Disposable Pod Kit – 18ml changeable pod with 650mAh rechargeable battery.

20K with Large Screen
20000 Puffs Disposable Vape with large screen. Normal and Boost working modes.

30K DTL Disposable
30K Puffs DTL(Directly to Lung) disposable vape with airflow control and screen.
Countries Allowing E-Cigarette Sales Under Regulation
While nearly four dozen countries ban sales, a larger number – 82 countries permit the sale of e-cigarettes but have implemented various regulatory controls. These regulations can be extensive and vary significantly, creating a complex compliance environment for international businesses. Common regulatory measures in these countries include:
- Age Restrictions: Virtually all countries allowing sales have minimum age limits for purchase, typically 18 or 21 years.
- Advertising, Promotion, and Sponsorship Bans: Many restrict or completely ban the advertising and promotion of e-cigarettes, similar to traditional tobacco products.
- Product Regulation: Rules concerning nicotine concentration, e-liquid volume, ingredients, and device safety standards.
- Packaging and Labeling: Requirements for health warnings, ingredient lists, and child-resistant packaging.
- Public Use Restrictions: Bans or limitations on vaping in public places, workplaces, and on public transport.
- Taxation: An increasing number of countries are imposing excise taxes on e-cigarettes and e-liquids.
- Retail Licensing: Requirements for businesses to obtain licenses to sell vaping products.
- Cross-Border Sales Restrictions: Rules governing online sales and importation by individuals.
Examples of countries in this category include most EU member states (which fall under the baseline EU Tobacco Products Directive), Canada, China, Australia (which has a unique prescription-only model for nicotine vapes), New Zealand, the United Kingdom (which heavily regulates but allows sales), and the United States itself.
The Unregulated Spaces
Despite the growing trend towards regulation or bans, a significant portion of the world still lacks specific e-cigarette laws. The WHO reported in 2023 that 74 countries, home to almost one-third of the world’s population (over 2 billion people), had no regulations in place addressing ENDS. This often includes many African and some Latin American countries where e-cigarettes may be found or are likely permitted simply due to the absence of specific laws disallowing them. This lack of regulation can lead to an unchecked proliferation of products of varying quality and safety, and often, unhindered marketing and sales to all age groups.
Full list here:

ECIGATOR
Ecigator is one of the well-known vape brands spun off from FM Technology Co., Ltd, it’s an ISO-certified disposable vape manufacturer for OEMs, ODMs, and OBM since 2010. The founder team comes from top firms with more than 10 years of experience in the vaping industry and has devoted thousands of hours to providing users with a better and better experience.
Key Considerations for Vape Businesses Venturing Internationally
For vape businesses aiming to export and sell vape products internationally in 2025, a meticulous approach to regulatory compliance is non-negotiable. Key steps include:
- Country-Specific Research: Do not assume that FDA compliance in the U.S. translates to compliance elsewhere. Each target market requires individual, in-depth regulatory assessment.
- Product Formulation and Design: Be prepared to reformulate e-liquids to meet nicotine strength caps (e.g., 20mg/mL in the EU/UK) and potentially remove or alter flavors based on local laws. Device designs (e.g., tank size limits of 2mL in the EU/UK) and battery rechargeability (relevant due to disposable vape bans) are also critical.
- Packaging and Labeling: Invest in market-specific packaging that complies with local language requirements, health warning sizes and content, ingredient listing formats, and child-resistant/tamper-evident standards.
- Notification and Registration: Understand and adhere to any pre-market notification or product registration processes required by national authorities (e.g., EU-CEG for EU states, MHRA portal for Great Britain).
- Advertising and Marketing: Develop marketing strategies that strictly comply with local advertising laws, which are often highly restrictive for vape products.
- Age Verification: Implement robust age verification processes for all sales channels, including online if permitted, to comply with local minimum age laws (typically 18 or 21).
- Stay Updated: The global regulatory landscape for vaping is highly dynamic. Continuously monitor for legislative changes in your target markets.
Navigating this complex environment often requires partnering with local legal and regulatory experts in each target country to ensure all nuances are addressed. The cost of non-compliance – including fines, product seizures, market access denial, and reputational damage – can be severe.
Read more:VAPE BANS: EXPLORING THE VAPE REGULATIONS IN U.S. AND WORLDWIDE
Conclusion
The global landscape for e-cigarette and vape regulation in 2025 is a mosaic of outright bans, stringent controls, and, in some regions, a concerning lack of specific oversight. For U.S. businesses, international expansion into this sector offers potential but demands an unwavering commitment to understanding and complying with a diverse and rapidly evolving set of rules. Prioritizing product safety, responsible marketing, and meticulous adherence to each target country’s specific legal requirements is paramount for sustainable success and for contributing positively to public health outcomes worldwide.
References
- WHO, ‘Electronic Cigarettes Call to action’ (2023). Available here.
- World Health Organization, “WHO report on the global tobacco epidemic, 2023: Protect people from tobacco smoke” (2023). Available here.
- DIRECTIVE 2014/40/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL. 2014. Available here.
- Challenges in domestic and regional courts to tobacco product ingredient regulation (WHO FCTC Article 9). WHO FCTC. (General reference to WHO FCTC guidelines influencing policy).
- Crosbie E, et al. (2024). Tobacco industry strategies to influence the regulation of new and emerging tobacco and nicotine products in Latin America and the Caribbean. Rev Panam Salud Publica. doi: 10.26633/RPSP.2024.43. (Cited by GGTC for Latin America).
- Malaysia Negeri Sembilan Backs Vape Ban, Awaits Clear Laws - August 5, 2025
- Is It Illegal to Vape or Smoke While Driving in Massachusetts? - August 5, 2025
- Austria Plans to Ban Disposable E-Cigarettes - August 5, 2025