Global Vape Compliance 2025: TPD, MHRA, PMTA & Certs
The global e-cigarette and vape market has witnessed explosive growth, transforming from a niche alternative into a multi-billion dollar industry with projections for continued expansion. For U.S. businesses eyeing international shores, this burgeoning market presents significant opportunities. However, venturing abroad with vape products in 2025 means stepping into an intricate and often fragmented web of international regulations. Understanding and meticulously adhering to these global vape regulations is no longer just advisable; it’s a fundamental prerequisite for successful market entry, sustained operations, and building a reputable international brand. This guide will illuminate the critical compliance aspects U.S. businesses must master, from decoding Europe’s TPD and the UK’s MHRA standards to appreciating the significance of product certifications and implementing robust internal compliance strategies.
The Evolving World of Vape Product Rules
Why does the rulebook for vape products often feel like it’s in a constant state of flux? The complexity arises primarily from the diverse public health strategies adopted by different nations. Some countries, like the UK, have historically embraced a harm reduction approach, viewing vaping as a significantly less harmful alternative for adult smokers struggling to quit combustible tobacco. Consequently, their regulations, while stringent on safety and youth access, might be geared towards ensuring adult smokers can access these alternatives. Conversely, other nations prioritize preventing youth initiation above all else, leading to much stricter controls, including comprehensive flavor bans or even outright sales prohibitions. This fundamental divergence in public health philosophy, coupled with varying product safety standards, ingredient restrictions, and marketing controls from one country to another, creates a challenging global picture for vape regulatory compliance.
For U.S. vape businesses, a superficial awareness of these international differences is insufficient. A deep, nuanced understanding is critical for successfully launching products in new territories and maintaining a positive brand image. Imagine the repercussions: a product perfectly legal and popular in one jurisdiction could face immediate recall, customs seizure, or hefty financial penalties in another due to a seemingly minor labeling oversight, an unapproved ingredient, or a non-compliant nicotine concentration. As we look at the 2025 landscape, the overarching global trend appears to be a move towards more stringent product standards, enhanced enforcement, and greater scrutiny on youth appeal. Therefore, mastering this dynamic regulatory environment is absolutely fundamental to your business’s viability, its reputation among consumers and international partners, and, most importantly, the safety of those who use your products. This foundational understanding of global vape regulations is the first step in navigating specific regional requirements.
Decoding Europe’s Tobacco Products Directive (TPD)
If your business has its sights set on the vast European Union market, the **Tobacco Products Directive (TPD)**, specifically Directive 2014/40/EU, is your primary regulatory framework. The TPD establishes baseline rules that apply across all EU member states for tobacco and related products, a category that explicitly includes e-cigarettes and e-liquids. A thorough understanding of its core tenets is non-negotiable for legal market access and operation.
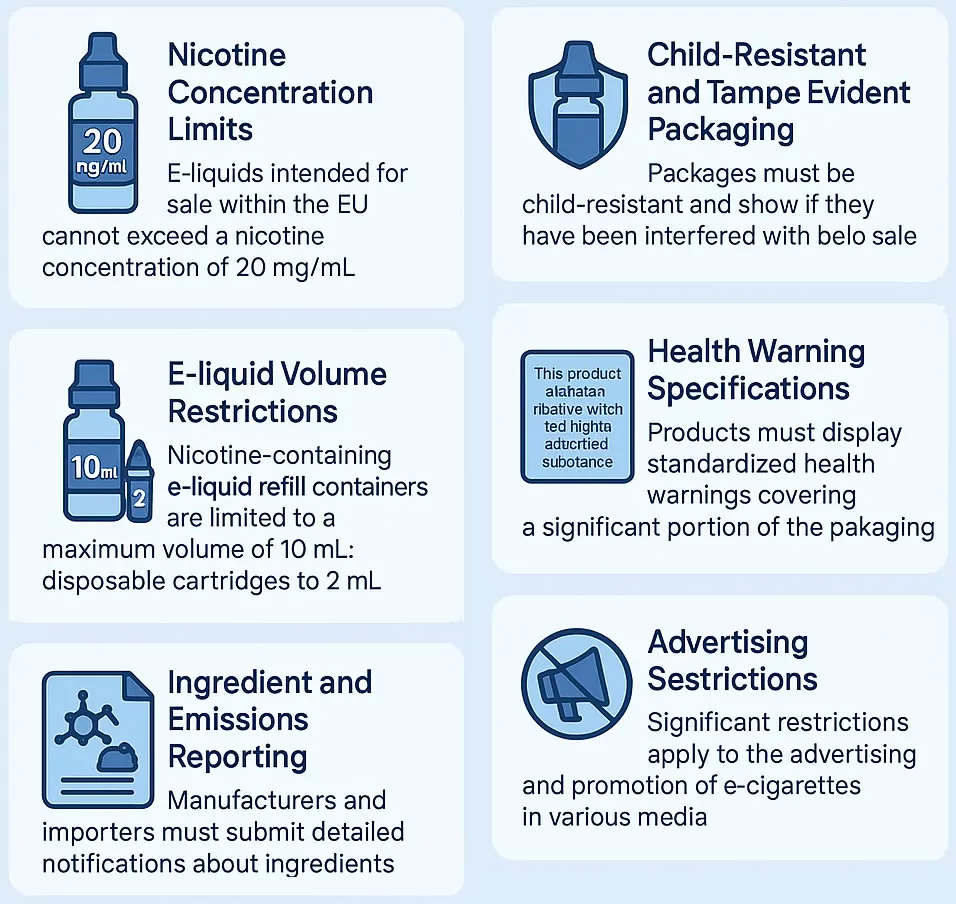
Core TPD Requirements for Vape Products
The TPD imposes several key mandates that directly influence product formulation, design, packaging, and marketing. These include:
- Nicotine Concentration Limits: E-liquids intended for sale within the EU cannot exceed a nicotine concentration of 20 mg/mL.
- E-liquid Volume Restrictions: Nicotine-containing e-liquid refill containers (e.g., bottles) are limited to a maximum volume of 10mL. For disposable e-cigarettes or single-use cartridges, the tanks or cartridges themselves must not exceed a capacity of 2mL.
- Child-Resistant and Tamper-Evident Packaging: All vape products containing nicotine must be sold in packaging that is both child-resistant (difficult for young children to open) and tamper-evident (showing if the product has been opened or interfered with before sale).
- Health Warning Specifications: Products must display specific, standardized health warnings, such as “This product contains nicotine which is a highly addictive substance.” These warnings must cover a significant portion of the packaging (e.g., 30% of the front and back surfaces) as dictated by the directive and individual member state implementations.
- Ingredient and Emissions Reporting: Manufacturers and importers must submit detailed notifications about their products to the competent authorities of the EU member states where they intend to sell. This includes information on ingredients, emissions from the product during use, and toxicological data. This is typically done via the EU Common Entry Gate (EU-CEG) portal, six months before placing new products on the market, as outlined in TPD Article 20.
- Advertising Restrictions: The TPD introduces significant restrictions on the advertising and promotion of e-cigarettes, particularly cross-border advertising in media like print, radio, television, and certain online platforms.
Impact on U.S. Businesses Targeting the EU Market:
These TPD vape guidelines profoundly shape how U.S. businesses must approach the EU market. For example, a popular U.S. e-liquid formulated at 50 mg/mL nicotine strength (common in some American products) would be illegal in the EU and require complete reformulation to meet the 20mg/mL cap. Product packaging designed for the U.S. market will almost certainly need redesigning to comply with EU warning sizes, languages, and child-proofing standards. Marketing strategies also need careful adaptation to align with the TPD’s advertising restrictions. Common pitfalls for businesses entering the EU include submitting incomplete or inaccurate notification dossiers through the EU-CEG system or misinterpreting the nuanced labeling and warning requirements, which can vary slightly in their presentation by member state, even under the harmonized directive.
Navigating Member State Variations
While the TPD establishes a harmonized framework, it’s crucial for U.S. businesses to remember that it sets minimum standards. Individual EU member states have the authority to implement additional, often stricter, national rules that go beyond the TPD’s baseline. These national variations can include:
- Complete bans on certain e-liquid flavors (beyond any potential future EU-wide restrictions).
- Specific national taxation levels for vape products (the TPD itself does not harmonize excise duties on vapes, leaving this to member states).
- Unique restrictions on domestic advertising or points of sale.
- Additional product registration or licensing fees at the national level.
This means that achieving general TPD compliance is only the first hurdle. Diligent, country-specific research into the national laws of each EU member state you plan to enter is absolutely essential to avoid costly fines, product seizures, or market access denial.

Ecigator Sticky Prefilled Pod Kit
The Ecigator Sticky Prefiiled Replaceable Vape Pod Kit is new kind of vape kit which the prefilled disposable pod can be changed.
That means you don’t need to throw away the whole kit but just change another pod. Also you can change the pods to taste different flavors.
Meeting UK’s MHRA Standards
Since the United Kingdom’s departure from the European Union (Brexit), U.S. businesses targeting the UK market (England, Wales, and Scotland – Great Britain, plus Northern Ireland which has a dual regulatory position under the Windsor Framework) must now adhere to a distinct set of regulations primarily overseen by the Medicines and Healthcare products Regulatory Agency (MHRA). While many core principles of UK regulation mirror the EU’s TPD due to their shared legislative history (the UK implemented the TPD via its Tobacco and Related Products Regulations 2016, which has since been amended post-Brexit), key differences in administrative processes and specific national rules demand careful attention.
Key MHRA Regulatory Aspects for Vapes:
The UK has established its own notification system for e-cigarette products, managed through the MHRA Submission Portal. Before vape products can be legally sold in Great Britain, businesses must submit detailed product information, including ingredients, emissions data, and toxicological reports. These MHRA vape standards are designed to ensure product safety and quality within the UK market. Northern Ireland has a unique position where EU TPD rules may still apply for goods placed on its market, requiring businesses to understand potential dual compliance needs if targeting the whole of the UK.
MHRA vs. TPD: Understanding Key Divergences
For a U.S. business planning to operate in both the EU and the UK, navigating the divergences between TPD and MHRA vape standards is critical. While core product parameters like the 20mg/mL nicotine strength limit and the 10mL maximum bottle size for nicotine-containing e-liquids remain consistent (as they were derived from the TPD), the administrative processes for market notification are entirely separate. You will need to manage distinct submissions to the EU-CEG for EU member states and to the MHRA portal for Great Britain. These may involve different data formatting requirements, submission fees, or review timelines. It is a mistake to assume that TPD compliance automatically ensures MHRA compliance, or vice versa. Each market requires its own dedicated due diligence and regulatory filings.
The Significance of UKCA Marking
A crucial change post-Brexit is the introduction of the UKCA (UK Conformity Assessed) mark. For many products placed on the market in Great Britain, including the electronic components of vape devices (which must meet electrical safety and electromagnetic compatibility standards), this mark is now mandatory, replacing the EU’s CE mark for this territory. Obtaining and correctly applying the UKCA mark signifies that your product meets all applicable UK regulatory requirements. Failing to display this mark correctly can lead to products being denied entry at customs, removed from shelves by Trading Standards, or other enforcement actions.
The following table provides a high-level comparison:
| Regulatory Aspect | TPD (European Union) | MHRA (United Kingdom – GB) | Implication for US Businesses |
|---|---|---|---|
| Governing Framework | EU Tobacco Products Directive 2014/40/EU | Tobacco and Related Products Regulations 2016 (as amended post-Brexit by UK law) | Compliance with two distinct, though related, legal frameworks is necessary. |
| Notification Portal | EU-CEG (EU Common Entry Gate) for individual member state notifications | MHRA Submission Portal for Great Britain | Requires separate product notification processes, accounts, and potentially fees. |
| Product Conformity Marking (for device electronics) | CE mark (if applicable under relevant EU directives like LVD, EMC) | UKCA mark (if applicable under relevant UK regulations) | Different conformity markings are needed for market access in Great Britain versus the EU. |
| Max E-liquid Bottle Size (nicotine-containing refills) | 10mL | 10mL | Consistent limit, simplifying one aspect of packaging for both markets. |
| Max Nicotine Strength | 20mg/mL | 20mg/mL | Consistent limit, simplifying e-liquid formulation for both markets. |
| Health Warnings | Standardized EU warnings, with member state language variations | UK-specific health warnings | Packaging must be adapted with market-specific warnings. |
This table outlines primary distinctions and some similarities between EU TPD and UK MHRA regulations relevant to vape products. U.S. businesses must address these distinct requirements when planning for European and UK market entry.

Ecigator OMIX 6000 (2+10ml) Pod Kit
The Ecigator OMIX 6000 is designed to be replaceable and rechargeable, and the ariflow is adjustable.
The kit comes with a 2ml prefilled pod and a 10ml E-liquid container, delivering up to 6000 puffs.
With the mesh coil technology, the OMIX 6000 will produces a consistent pure taste to ensure a superior vaping experience.
Understanding FDA Oversight in the United States
While your primary goal might be international expansion, a brief understanding of the U.S. Food and Drug Administration’s (FDA) regulatory approach to vaping products provides essential context. The complexities and demands of the U.S. system can often serve as a primer for the varied and stringent regulatory landscapes you might encounter abroad. Mastering your home market’s vape regulatory compliance requirements helps frame the global challenge.
In the United States, the FDA regulates e-cigarettes, vapes, and other ENDS as “tobacco products.” This authority grants the FDA control over many aspects, including manufacturing standards, ingredient reporting, marketing, and sales. The overarching aim is public health protection, with a very significant focus on preventing youth access and nicotine addiction.
The cornerstone of FDA regulation for any new vape product (or existing product with significant modifications) is the Premarket Tobacco Product Application (PMTA) process. This is the primary legal pathway for bringing new vape products to the U.S. market and is an incredibly rigorous, time-consuming, and resource-intensive undertaking. The PMTA pathway requires applicants to submit extensive scientific evidence demonstrating that permitting the marketing of their new tobacco product would be “appropriate for the protection of public health.” This involves detailed studies on product chemistry, manufacturing consistency, toxicological profiles, health impacts on users and non-users, and the likelihood of youth initiation versus adult smoker cessation. Many businesses, especially smaller ones, find the PMTA process to be a formidable barrier due to its complexity and substantial cost.
Looking at 2025, FDA enforcement is expected to remain robust, particularly concerning unauthorized flavored vape products, especially disposable e-cigarettes that are often deemed appealing to youth and lack marketing authorization. Beyond federal FDA regulations, U.S. businesses also contend with a complex patchwork of state and local laws. These can include state-specific flavor bans (e.g., California, Massachusetts), varying excise taxes on vape products, and differing sales or public use restrictions, adding multiple layers of complexity even within the domestic market. This multi-layered system in the U.S. offers a valuable glimpse into the kind of regulatory diversity and intensity you might encounter when venturing into multiple international markets.
Ecigator is one of the well-known vape brands spun off from FM Technology Co., Ltd, it’s an ISO-certified disposable vape manufacturer for OEMs, ODMs, and OBM since 2010. The founder team comes from top firms with more than 10 years of experience in the vaping industry and has devoted thousands of hours to providing users with a better and better experience.

18K Disposable Pod Kit
Disposable Pod Kit – 18ml changeable pod with 650mAh rechargeable battery.

35K with Large Screen
35000 Puffs Disposable Vape with 3D galaxy screen. Eco and Pulse working modes.

30K DTL Disposable
30K Puffs DTL(Directly to Lung) disposable vape with airflow control and screen.
The Value of Product Certifications (CE, ROHS, UKCA)
Beyond adhering to overarching regulatory frameworks like the TPD or MHRA standards, specific product certifications play a vital role in demonstrating compliance, ensuring safety, and building trust in international markets. For U.S. businesses, understanding and obtaining these vape product certifications is not merely about ticking legal boxes; it is about signaling a profound commitment to quality and safety that resonates powerfully with both discerning consumers and international business partners.

CE Marking: European Economic Area (EEA) Conformity
The CE mark is a mandatory conformity marking for a wide range of products sold within the European Economic Area (which includes all EU countries plus Iceland, Liechtenstein, and Norway). It signifies that the manufacturer has declared the product conforms with all applicable EU health, safety, and environmental protection standards. For vape devices, particularly their electronic components (batteries, chargers, circuitry), CE marking is crucial. It demonstrates that your device meets essential EU requirements, such as those under the Low Voltage Directive (LVD) and the Electromagnetic Compatibility (EMC) Directive. Without a valid CE mark, your electronic vaping product cannot be legally placed on the EEA market.
ROHS Compliance: Restricting Hazardous Substances in Electronics
ROHS stands for the Restriction of Hazardous Substances. This EU directive (now also adopted in similar forms by many other regions, including the UK) restricts the use of specific hazardous materials found in electrical and electronic equipment. These restricted substances include lead, mercury, cadmium, hexavalent chromium, polybrominated biphenyls (PBBs), and polybrominated diphenyl ethers (PBDEs). For vape devices, which are electronic products, ROHS compliance is essential. It ensures that your products are manufactured using safer materials, thereby reducing their environmental impact at end-of-life and minimizing potential health risks for consumers from exposure to these hazardous substances. Many international markets beyond the EU now look for ROHS compliance as a benchmark for product safety and environmental responsibility.
UKCA Marking: Your Key to the Great Britain Market
As highlighted earlier, the UKCA (UK Conformity Assessed) mark is the conformity marking required for most goods being placed on the market in Great Britain (England, Wales, and Scotland) that previously required the CE mark. For vape devices and their electronic components sold in Great Britain, ensuring your products meet UKCA requirements is mandatory. This demonstrates that your product complies with all relevant UK legislation, which, while often similar to EU rules, now has its own distinct legal standing and administrative processes. The UKCA mark is a clear visual indicator of this compliance.
Securing these vape product certifications offers broader strategic benefits beyond mere legal market entry:
- Enhanced Market Access: These certifications are often non-negotiable prerequisites for entry into major international markets.
- Increased Consumer Trust and Confidence: Visible conformity marks like CE and UKCA signal to consumers that the products they are purchasing meet recognized and robust safety standards.
- Demonstration of Product Quality and Safety: They provide tangible, verifiable proof of your company’s commitment to high manufacturing standards and consumer protection.
- Reduced Risk of Regulatory Complications: Proper certification significantly minimizes the chances of customs delays, product recalls, fines, or other enforcement actions.
- Stronger Business Partnerships: International distributors and retailers are more likely to partner with businesses whose products carry recognized certifications, as it reduces their own compliance risks.
Consider these certifications as your product’s essential travel documents for international markets, assuring authorities, partners, and consumers alike of their adherence to critical safety and quality benchmarks.

ECIGATOR
Ecigator is one of the well-known vape brands spun off from FM Technology Co., Ltd, it’s an ISO-certified disposable vape manufacturer for OEMs, ODMs, and OBM since 2010. The founder team comes from top firms with more than 10 years of experience in the vaping industry and has devoted thousands of hours to providing users with a better and better experience.
Practical Steps for Ensuring Global Regulatory Adherence: A Proactive Approach
Understanding the myriad of international rules is a significant first step; consistently adhering to them in a dynamic global market is an ongoing operational challenge. For U.S. businesses, especially those relatively new to exporting complex regulated products like vapes, establishing a systematic and proactive approach to vape regulatory compliance is absolutely essential for sustainable long-term success. This is not a one-time task but a continuous commitment that must be embedded within your business culture and operations.
Here are some practical strategies to effectively manage the complexities of global vape regulations:
- Develop a Robust Internal Compliance Program: This is your foundational strategy. Establish clear internal systems and assign responsibilities for tracking changes in international vape laws and standards. This program should encompass strict quality control measures at every stage of product design, component sourcing, and manufacturing. Meticulous documentation practices for all compliance-related activities are vital. Clearly define who within your organization is responsible for monitoring updates from agencies like the MHRA, individual EU member state authorities, or other target market regulators.
- Prioritize Comprehensive Product Testing and Meticulous Record Keeping: You cannot simply assert that your product is safe and compliant; you must be able to demonstrate it with credible evidence. Comprehensive testing for e-liquid ingredients, emissions from the aerosol, battery safety, electrical safety of the device, and material safety is vital. Maintain detailed and organized records of all such tests, as well as batch production records, supplier certifications (e.g., for ROHS compliance of components), and quality control checks. This documentation will be invaluable if regulators ever question your product’s compliance or in the event of a product issue.
- Do Not Hesitate to Seek Specialized Expert Help: The world of international vape regulations is intricate, highly technical, and constantly evolving. If your internal team lacks the specific expertise or bandwidth to navigate these complexities effectively, seriously consider partnering with specialized regulatory consultants, testing laboratories, or legal experts who focus on tobacco and vape product compliance in your target markets. Their in-depth knowledge and experience can save you significant time, resources, and potential legal headaches in the long run. An expert eye can often identify potential compliance gaps or upcoming regulatory shifts that your team might overlook.
- Manage Labeling and Packaging Variations Systematically and with Precision: Different international markets invariably mean different rules for health warnings (content, size, placement, language), ingredient lists, nicotine content declaration, symbols, and even packaging dimensions or child-resistance standards. Implement a robust version control system for all your labeling artwork and packaging designs to ensure that the correct, fully compliant version is used for each specific market. A simple labeling or packaging mix-up can lead to entire shipments being rejected at customs or products being recalled from the market.
- Stay Actively Updated and Engaged: Regulatory landscapes are not static; they are living entities that change in response to new scientific evidence, public health concerns, and political priorities. Make it a routine operational practice to actively monitor official sources of regulatory information. Subscribe to reputable industry newsletters that cover international regulatory developments, regularly check the websites of key regulatory agencies like the FDA (for U.S. context), the MHRA (for the UK), the European Commission’s public health directorate (for EU-wide TPD updates), and the national health or consumer protection authorities in your specific target countries. Participating in relevant industry forums or trade associations can also provide valuable early warnings and insights into potential regulatory changes. What was compliant yesterday might require adjustment or re-notification tomorrow.
Ultimately, a proactive, systematic, and well-resourced approach to vape regulatory compliance is not merely about avoiding penalties or legal trouble. It is about building a sustainable, ethical, and reputable international brand that consumers, regulators, and business partners can trust. This unwavering commitment to compliance and safety is a cornerstone of successfully and responsibly operating in the global vape market of 2025 and beyond.









